High Performance Catalysts for Wastewater Treatment

The header image was purchased on Istock.com and is protected by copyright.
Due to increasing volumes of wastewater and its growing toxicity, photocatalysis is a promising solution that relies on the ability of semiconductor materials to absorb light and to produce chemical reactions to degrade pollutants. Photocatalysis can process and destroy water contaminants, such as antibiotics, hormones and other endocrine disruptors, well known for their harmful effects on fauna and flora. Because the technology used in wastewater treatment plants is incomplete, pollutants are discharged as is. Using innovative catalysts, we aim to improve the integration of this technology in wastewater treatment plants. Also, we strive to use industry-compatible production methods for the large-scale production of the proposed materials.
Photocatalysis – How Does it Work?
In wastewater treatment, the aim of photocatalysis is to transform water pollutants into harmless substances, a process called pollutant degradation. To reach this objective, catalysts are used. In our case, we use semiconductor materials capable of absorbing light and generate electron-hole pairs. If they reach the catalyst surface and encounter the liquid to be processed, these pairs can participate in chemical reactions and thus degrade the pollutants.
What Are the Challenges of Photocatalysis?
In practice, the challenges of photocatalysis are primarily related to the intrinsic properties of the semiconductor materials used as catalysts.
Firstly, most of the efficient catalysts used today, such as titanium dioxide or zinc oxide, are only able to generate electron-hole pairs through the absorption of ultraviolet (UV) light. Dangerous to humans, UV light represents only 3% to 5% of the solar irradiation that reach the surface of the Earth. Therefore, the efficiency of these materials is limited in the presence of natural light. One solution is to use large reactors exposed to UV lamps. However, the use of UV sources which are expensive, restrictive and dangerous for the operators substantially limits the integration of these materials in wastewater treatment plants.
Secondly, in order to facilitate the contact of electron-hole pairs with water and maximize the photocatalytic effect, the size of the catalysts must be optimized. Generally catalysts are used in the form of microscopic or even nanometric particles, usually in suspension. Once photocatalysis has been performed, these particles must be recovered to obtain contaminant-free treated water and to ensure a competitive treatment cost. This constraint complicates the integration of these materials on a larger scale.
Our Strategies to Improve Photocatalysis
In our research, we propose simple, industry-compatible methodologies that eliminate the need for UV sources and recovery procedures [1] [2]. We focus our efforts on bismuth ferrite or BiFeO3 (BFO), a material capable of absorbing visible light. Through electrospinning, we synthesize membranes composed of BFO fibres. Compared to the traditionally-used suspended particles, these fibers are firmly attached to a glass slide. This approach eliminates the constraint of recovering the catalyst after pollutant degradation.

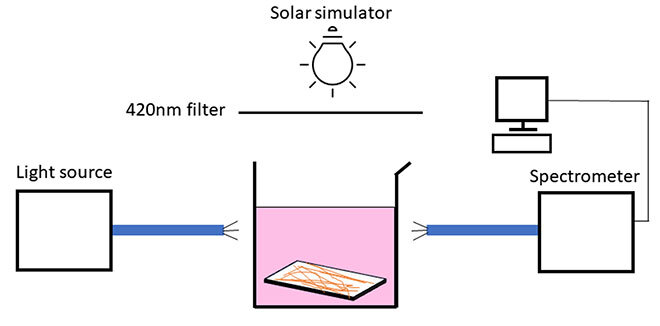
Figure 1 – Beaker containing rhodamine B and a BFO fibre membrane. The bubbles are the result of chemical reactions occurring at the membrane surface.
We also demonstrate that the size and deposit time of the BFO fibres must be optimized in order to obtain the highest pollutant degradation. In our studies, the pollutant molecule is a dye called rhodamine B. This dye is used to track water flows but is harmful to aquatic organisms. We demonstrated that 92% of the initial pollutant concentration degrades in 2.5 hours under UV-free artificial light. The degradation of rhodamine B is maintained above 80% even after five cycles of use. Moreover, the physical integrity of the membrane is preserved at more than 99%.
Most importantly, this membrane is environment-friendly, as it involves a biocompatible material and requires roughly 100 times less material than state-of-the-art approaches for similar degradation performance. Manufactured by industry-ready techniques, this membrane provides hope for a large-scale deployment of the developed membrane. In our research, a similar membrane was also produced using manufacturing-ready screen-printing strategies. It relies on synthesized BFO powder films on which another semiconductor can grow. This membrane benefits from a synergy between both materials and offers another solution to avoid catalyst recovery at the end of the pollutant degradation process.
Outlook
Following the publication of these results, we are continuing our efforts to always produce more efficient photocatalytic membranes. To do so, we are exploring the synergy between BFO fibres and titanium dioxide nanoparticles. We have obtained promising preliminary results. In order to validate our work on a larger scale, we must study the degradation of different pollutants involving larger volumes to be processed. This new initiative is also supported through the ÉTS-FRECC initiative to fight climate changes.
Additional Information
For more information on this research, please see the following papers:
Fourmont, P., Nechache, R., & Cloutier, S. G. (2021). Reusable BiFeO3 Nanofiber-Based Membranes for Photo-activated Organic Pollutant Removal with Negligible Colloidal Release. ACS Applied Nano Materials, 4(11), 12261-12269.
Fourmont, P., & Cloutier, S. G. (2022). Screen-printed p–n BiOCl/BiFeO3 heterojunctions for efficient photocatalytic degradation of Rhodamine B. RCS Advances, 9(?? ), 24868-24875.